Patients with univentricular heart physiology are palliated by staged surgical corrections to direct blood from the caval veins to the pulmonary arteries without the interposition of a subpulmonary ventricle (Fontan circulation). These patients are frequently suffering from progressively deteriorating hemodynamics while being left without adequate therapeutic options. In our group, we address this unmet medical need by translating key clinical demands toward a novel implantable pump to substitute the missing ventricle. Pre-clinical evaluation of the proposed technology showed the potential for efficient, low-traumatic chronic support of an inclusive patient population. This pre-clinical research denotes the inevitable first step towards durable and reliable treatment strategies to restore biventricular equivalency in Fontan patients and accordingly improve their quality of life.
Research Group Leader
ap. Prof. Marcus Granegger, PhD
Post-Doc Research Associates
Dr.-Ing. Bente Thamsen
PhD-Candidates
Barbara Karner, MD
Pascal Schmidt, MSc
The healthy human heart comprises both a right ventricle, responsible for pumping blood through the lungs, and a left ventricle, which pumps blood throughout the body. However, approximately 1 in 3000 live births suffer from a condition known as a univentricular heart, where one of the ventricles is underdeveloped, leaving only one functional ventricle. These patients typically undergo a series of multi-stage surgeries to connect the caval veins to the pulmonary arteries in a total cavopulmonary connection (Figure 1A). In the resulting Fontan circulation, the sole functional ventricle takes on the role of circulating the blood, often resulting in adverse hemodynamics characterized by venous congestions and associated complications.
Fontan circulation - Assisted Fontan circulation
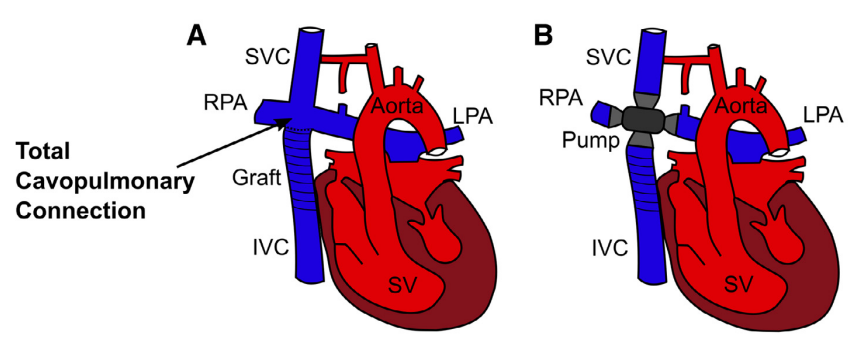
Our objective is to develop a rotodynamic blood pump designed for implantation into the total cavopulmonary connection. This pump will serve to substitute the absent subpulmonary ventricle, thereby restoring biventricular hemodynamics (Figure 1B).
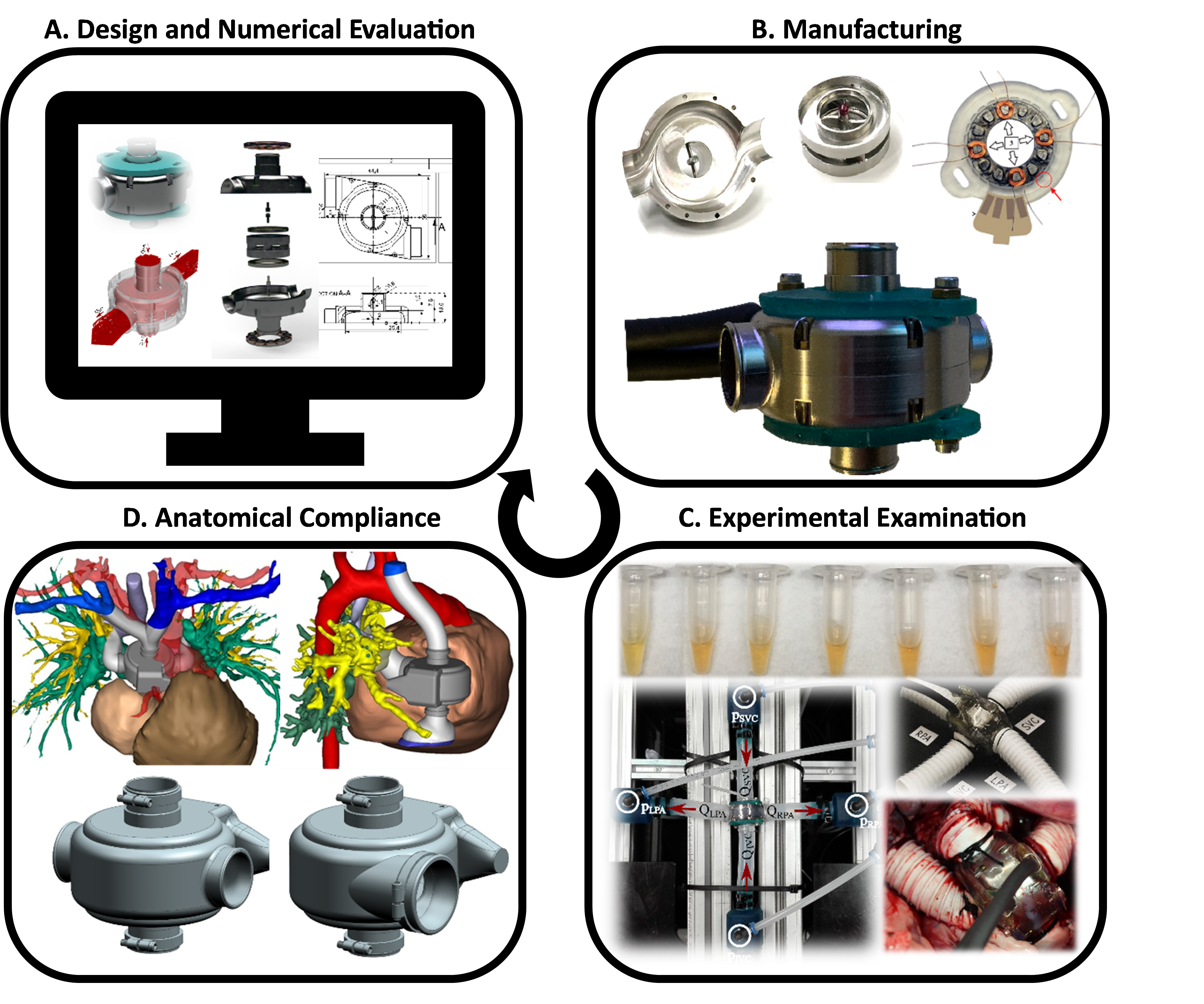
The development of the novel cavopulmonary assist device involves a multitude of different methods from various disciplines including lumped-parameter modelling, computational fluid dynamics simulation, in-vitro hemocompatibility evaluation and in-vivo animal trials (Figure 2).
To date, we have conducted comprehensive preclinical assessments, including acute in-vivo animal trials. The examination revealed the potential for broad-range operation with excellent hemocompatibility and low power consumption. In-vivo trials have confirmed the feasibility of restoring biventricular hemodynamics. Currently, we are leveraging the insights gained from the preclinical evaluation of the first-generation device to inform the design of the second-generation device.
Video - Preclinical development and testing of a novel cavopulmonary assist device.
Reproduced with permission from Escher et al. 2021,
Copyright © 2021 The Author(s). Published by Elsevier Inc
Given the anticipated doubling of the Fontan population in the decades to come, the limited availability of donor hearts and the complexity of cardiac transplantations in these patients, a mechanical assist device is urgently needed for patients suffering from Fontan failure. Such a device will be key to alleviate severe side-complications in Fontan patients and thus to promote quality of life in this population.
The project is funded by the GIGAX foundation, the Ever foundation and the 4Fontan AG.
Cooperation partners
University Medical Center Hamburg-Eppendorf (UKE) (Prof. Michael Hübler, Prof. Jörg Sachweh)
ETH Zurich (Prof. Johann Walter Kolar, Dr. Jonas Huber)
University of Innsbruck (Ass. Prof. Spasoje Miric)
Technische Universität Berlin (Prof. Paul Uwe Thamsen)
Leibniz University Hannover (Dr. Marc Müller)
Publications
- Karner B, Escher A, Schorn T, Narayanaswamy K, Sachweh J, Laufer G, Hübler M, Zimpfer D, Granegger M. Anatomical Compliance of Cavopulmonary Assist Device Designs – A Virtual Fitting Study in Fontan Patients. ASAIO J, accepted for publication, July 2023.
- Granegger M, Escher A, Karner B, Kainz M, Schlöglhofer T, Schwingenschlögl H, Roehrich M, Podesser BK, Kramer AM, Kertzscher U, Laufer G, Hübler M, Zimpfer D. Feasibility of an Animal Model for Cavopulmonary Support with a Double-Outflow Pump. ASAIO J. 2023;69(7):673-680. doi:10.1097/MAT.0000000000001916
- Escher A, Strauch C, Hubmann EJ, Hübler M, Bortis D, Thamsen B, Mueller M, Kertzscher U, Thamsen PU, Kolar JW, Zimpfer D, Granegger M. A Cavopulmonary Assist Device for Long-Term Therapy of Fontan Patients. Semin Thorac Cardiovasc Surg. 2021;34(1):238-248. doi:10.1053/j.semtcvs.2021.06.016
- Strauch C*, Escher A*, Wulff S, Kertzscher U, Zimpfer D, Thamsen PU, Granegger M. Validation of numerically predicted shear stress-dependent dissipative losses within a rotary blood pump. ASAIO J. 2021;67(10):1148-1158. doi:10.1097/MAT.0000000000001488
- Granegger M, Thamsen B, Hubmann E, Choi Y, Beck D, Valsangiacomo E, Voutat M, Schweiger M, Meboldt M, Hübler M. A long-term mechanical cavopulmonary support device for patients with Fontan circulation. Medical engineering & physics 2019;70:9-18. doi: https://pubmed.ncbi.nlm.nih.gov/31266678/
- Hubmann E, Bortis D, Flankl M, Kolar JW, Granegger M, Hübler M. Optimization and calorimetric analysis of axial flux permanent magnet motor for implantable blood pump assisting the Fontan circulation. In 2019 22nd International Conference on Electrical Machines and Systems (ICEMS) (pp. 1-8). IEEE. doi:https://dl.acm.org/doi/10.1109/ICEMS.2019.8921580